
Home » Two-Thirds of Trial Subjects for Drug Approvals Are Outside the U.S.
Two-Thirds of Trial Subjects for Drug Approvals Are Outside the U.S.
November 16, 2020
Almost two-thirds of participants in clinical trials to support U.S. drug approvals from 2015 to 2019 were located outside the U.S., the FDA’s Center for Drug Evaluation and Research (CDER) said in a new report released last week.
The three countries that furnished the largest number of the 292,766 study participants — after the U.S. with its 102,596 participants — were Poland, Germany and Russia at about 4 percent of the total each, followed by Japan, Canada and the Czech Republic at about 3 percent each, according to CDER’s five-year summary of Drug Trials Snapshots.
Among U.S. states, California, Florida and Texas had the largest number of study participants, at 14,525, 12,038 and 8,709, respectively.
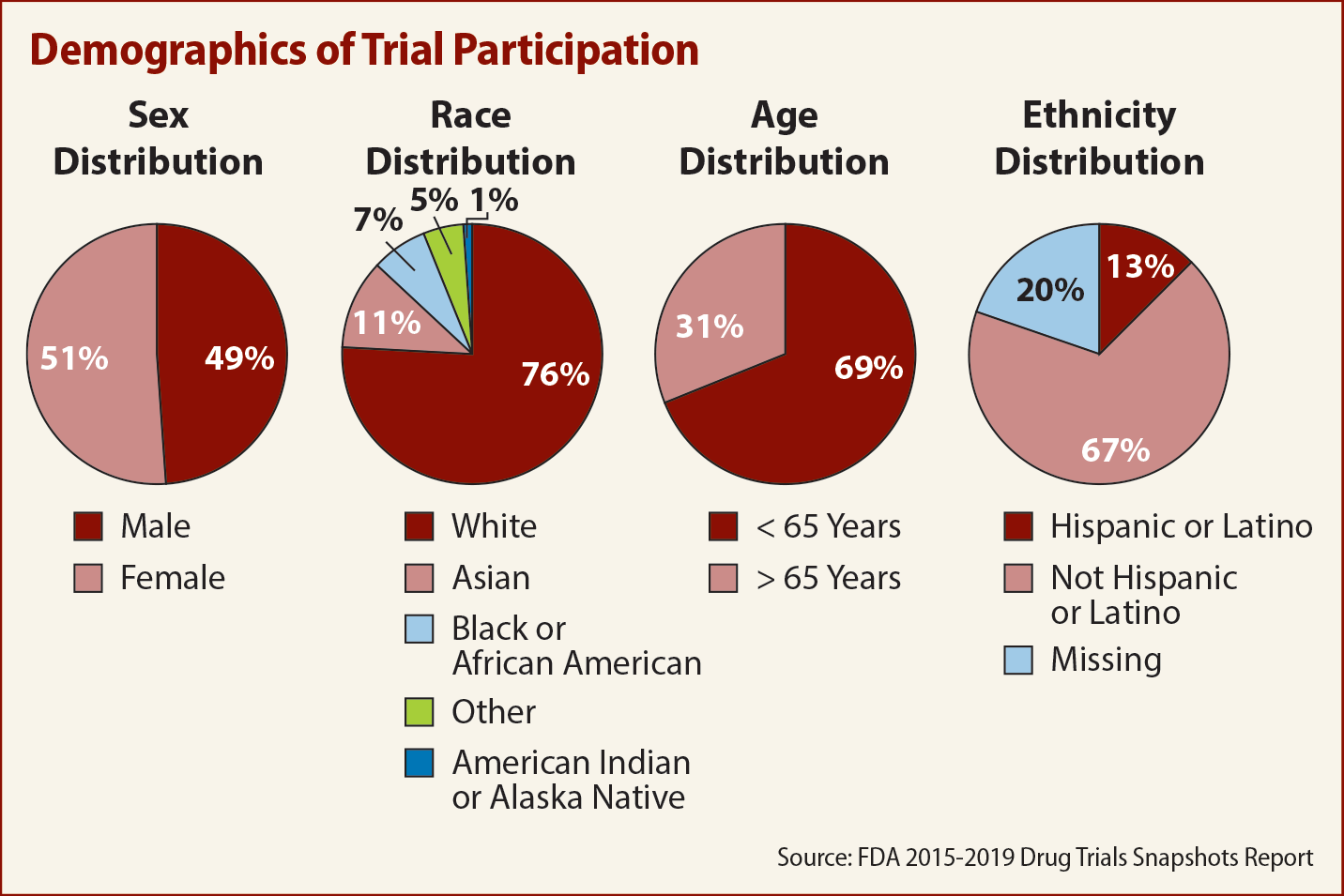
Demographic analysis of the trial participants showed a significant tilt toward females in the U.S., at 56 percent of the total, while outside the U.S., aggregate female participation was only 49 percent.
The report found that most Black or African American participants were from U.S. sites. In the U.S., 16 percent of trial participants were Black or African American, against only 2 percent in the rest of the world, which lowers the global participation rate for this racial group to just 11 percent. As of July 1, 2019, 13.4 percent of the U.S. population was African American, according to the U.S. Census Bureau, so the 11 percent global figure means this racial group was underrepresented.
Race distribution by age was similar, but the proportion of Black or African Americans in the 65-and-older age group was 6 percent lower.
At 78 percent of trial participants, white people were slightly overrepresented compared to their 76.3 percent share of the U.S. population, a figure that includes some Latinos, which the U.S. Census Bureau classifies as an “ethnicity” and not a racial group.
In other notable findings:
- Between 39 percent and 58 percent of all approvals over the five-year period were for drugs treating rare diseases.
- Cardiovascular diseases accounted for the largest number of trials, followed in second place by endocrinology and metabolism, and by oncology and hematology in third place.
- The majority of trial participants were younger than 65 years of age. On average, participants in the U.S. tended to be younger than those in the rest of the world.
Read the full Drug Trials Snapshots report here: https://bit.ly/3kvjpX1.




